Business Process & Interactions in Practice – End Users
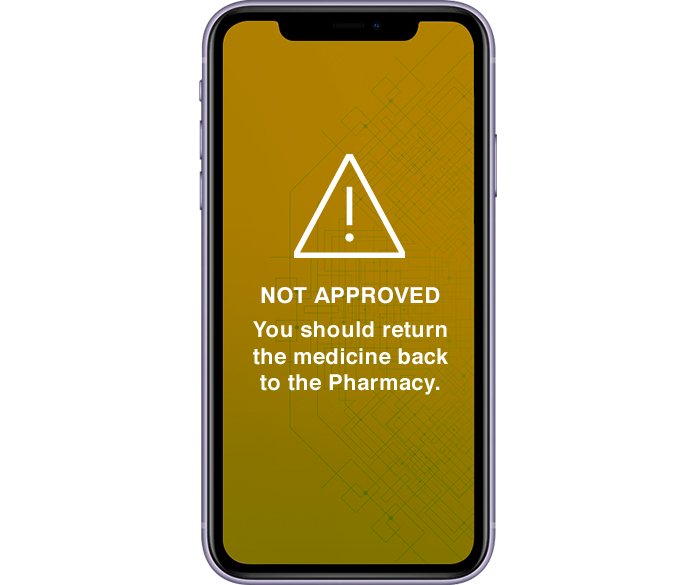

Product validation and reporting of counterfeits, expired or grey market medicines.

Validate and confirm release of approved drugs and devices.

Scan a barcode of drugs or devices with their mobile device to capture and verify real time supply chain data.

Product validation on arrival and upon dispensing medicine.

